
Cross & Dot PDF download
Representing Dot & Cross Diagrams. Dot and cross diagrams are diagrams that show the arrangement of the outer-shell electrons in an ionic or covalent compound or element. The electrons are shown as dots and crosses. In a dot and cross diagram: Only the outer electrons are shown. The charge of the ion is spread evenly which is shown by using.
13+ Dot And Cross Diagram Robhosking Diagram
Awesome "crucifix" & High Quality Here On Temu. New Users Enjoy Free Shipping & Free Return. Only Today, Enjoy "crucifix" Up To 90% Off Your Purchase. Hurry & Shop Now
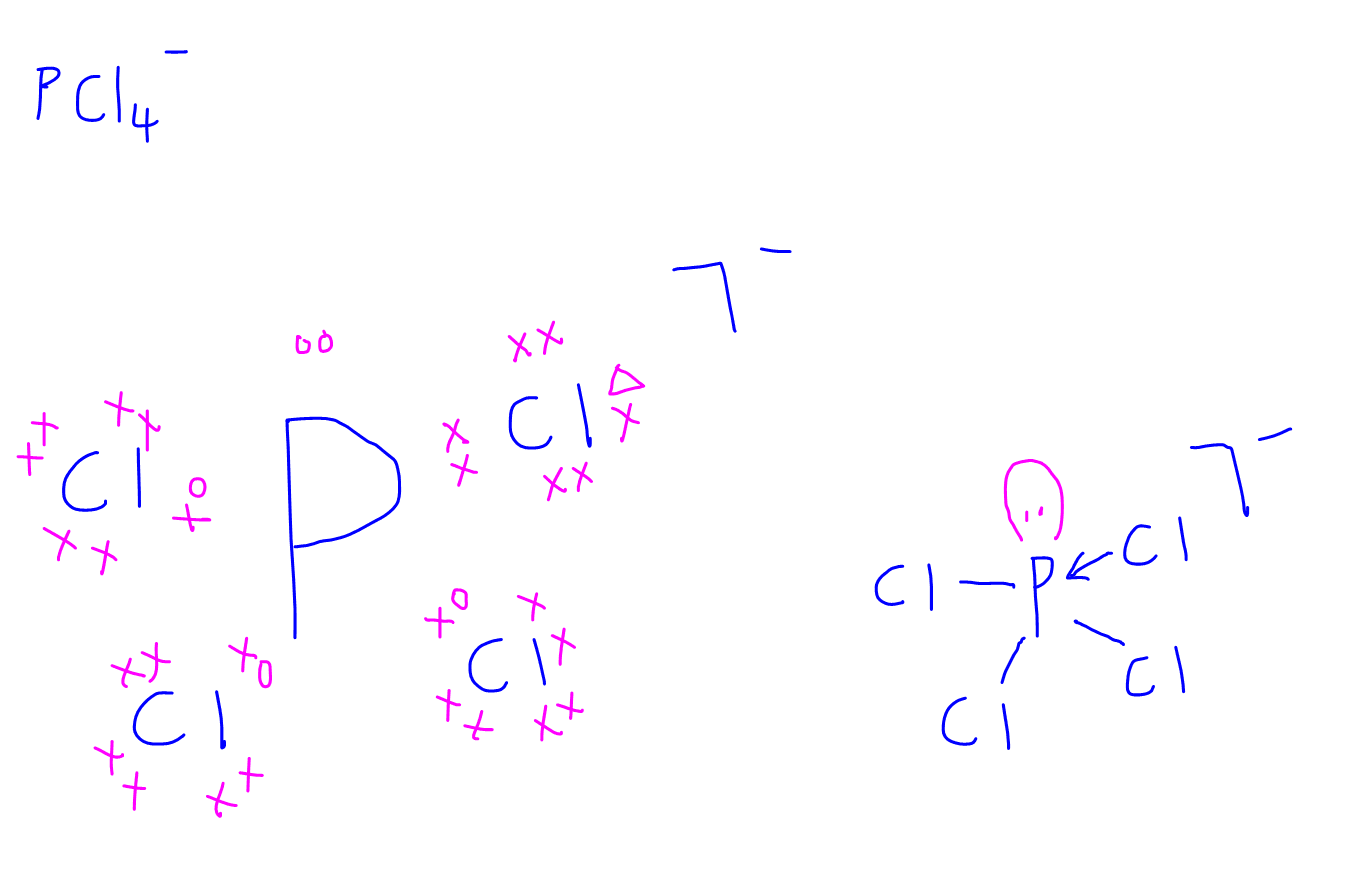
Physical Chemistry Video Lessons
Dot- and- cross diagram of covalent molecule carbon dioxide (CO 2) Let's look at drawing the dot-and-cross diagram of carbon dioxide. Oxygen is in group VI of the periodic table. With 6 valence electrons, it needs 2 more electrons. So it will share 2 electrons to achieve stable octet configuration. It can share the 2 electrons with 1 atom.
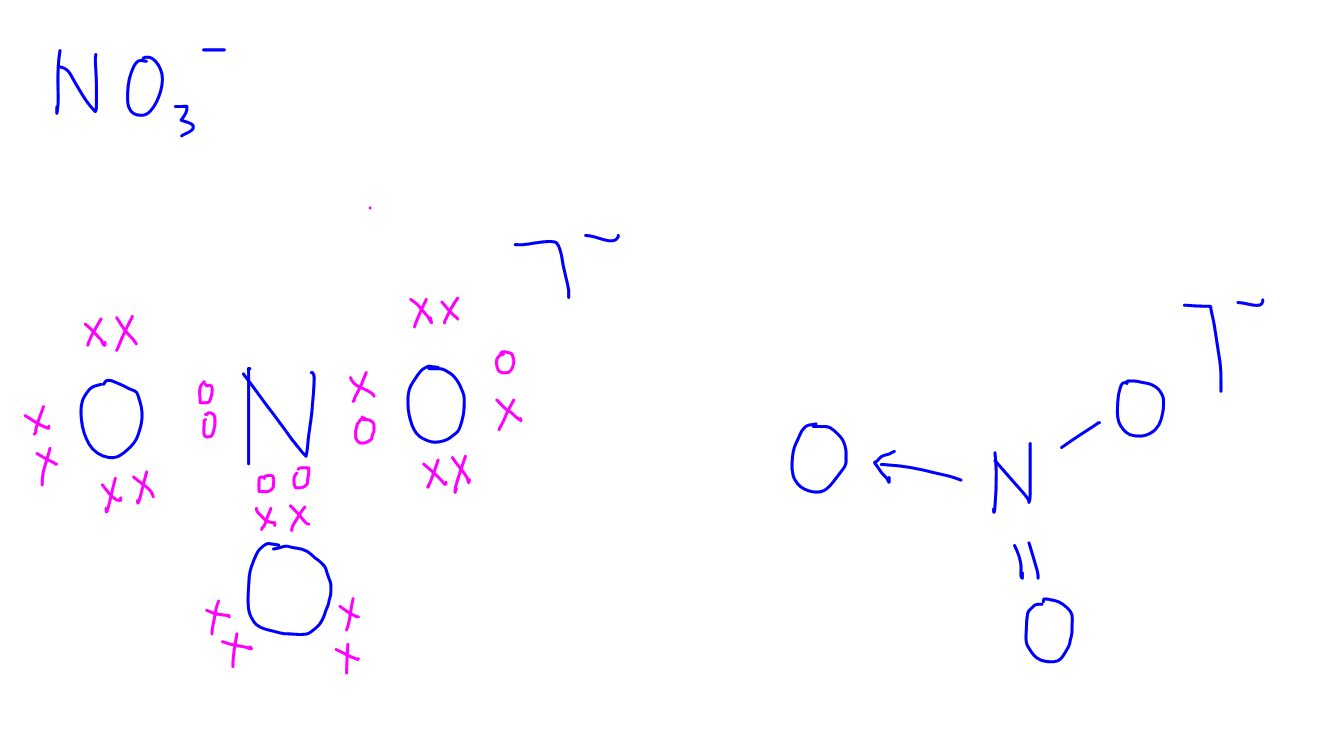
Physical Chemistry Video Lessons
In dot and cross diagrams, commonly introduced at 14-16 (Figure 1b), each atom's outer shell electrons are shown in circular orbits. Covalent bonds are represented by a shared pair of electrons in an area of overlap. Neither of these models show the 3D shape of the molecule.
.PNG)
Bohr’s Atom
A dot and cross diagram can model the bonding in a simple molecule : the outer shell of each atom is drawn as a circle circles overlap where there is a covalent bond electrons from one atom are.

Dot & Cross Paper 91cmx150m
In this video we want to learn how to draw dot and cross diagrams for molecules and ions in Chemical Bonding.Drawing dot-cross diagram is a fundamental and e.

Dot cross (Dotcross2) Twitter
In a dot and cross diagram, ions are drawn as a central nucleus, surrounded by rings of orbiting electrons (represented by either dots or crosses). The formation of ionic molecules can be shown by using dots to represent the electrons of one ion, and crosses for the electrons of another. By placing the dot of a metal ion in the outer ring of.

Dot and Cross Products YouTube
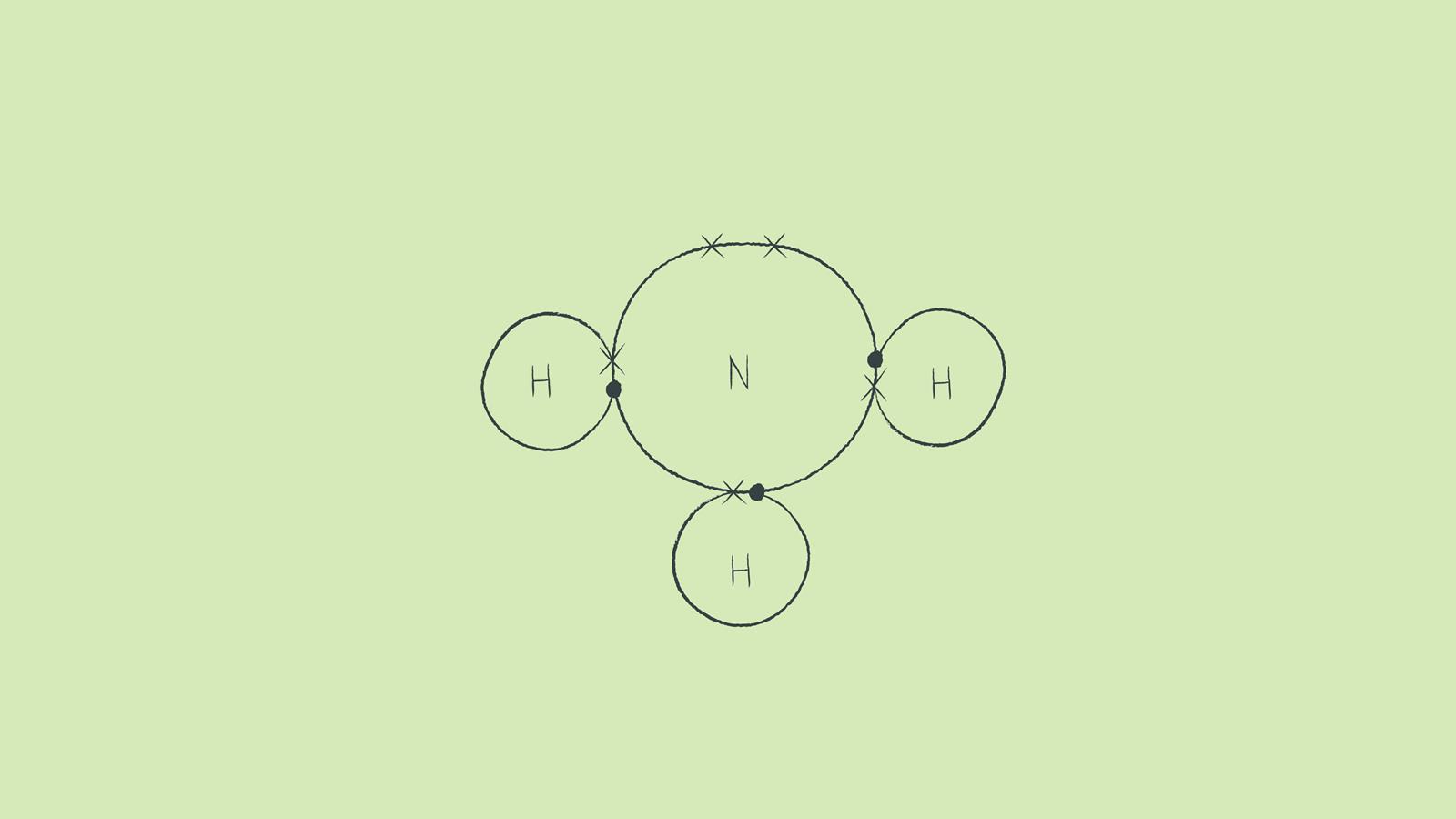
How to draw a dot and cross diagram for ammonia In NH 3, commonly known as ammonia, nitrogen forms three single covalent bonds with three hydrogen atoms. Source: © Dan Bright While you are learning how to draw dot and cross diagrams it's useful to start with something you are already familiar with: electron configuration diagrams.

Drawing Dot and Cross Diagrams
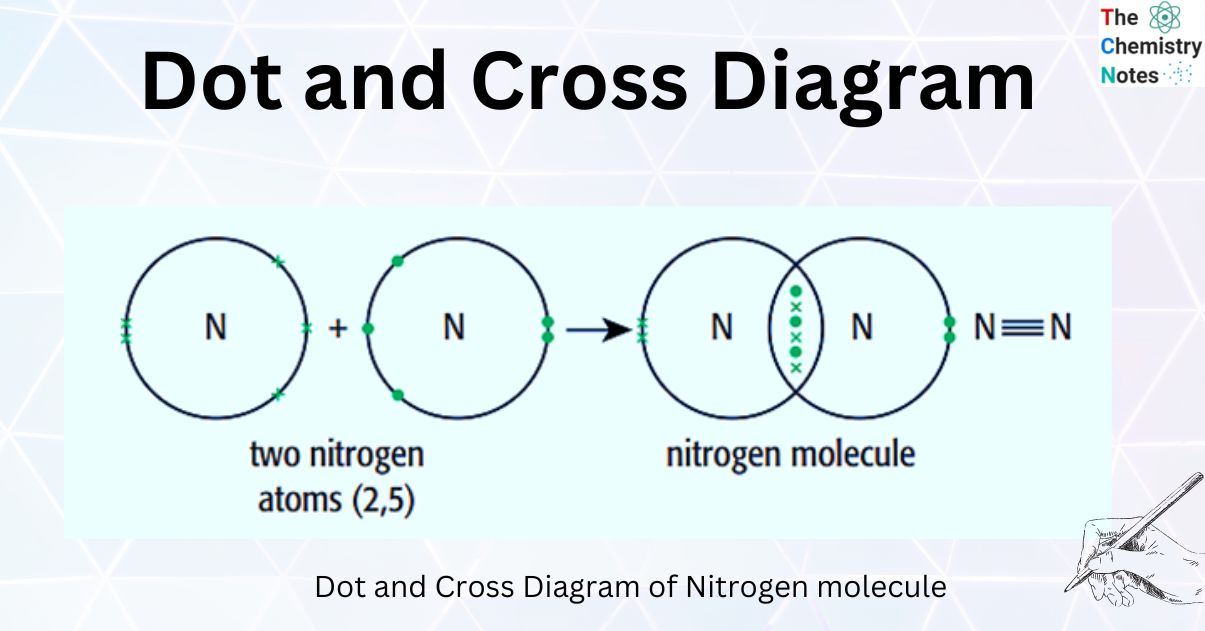
Dot-and-cross diagrams of covalent compounds in which the atoms share their valence electrons Double covalent bonding Oxygen, O2 Covalent bonding in oxygen Carbon dioxide, CO2 Covalent bonding in carbon dioxide Ethene, C2H4 Covalent bonding in ethene Triple covalent bonding Nitrogen, N2 Covalent bonding in nitrogen Dative covalent bonding
33 best ideas for coloring Dot And Cross Diagram
Dot and cross models show how a pair of electrons form a covalent bond . Notice that in the diagrams, only the electrons in the outer shell of each are shown. Examples of dot and cross models.
Cross dot to dot printable worksheet Connect The Dots
A dot and cross diagram can show the bonding in a small molecule: the outer shell of each atom is drawn as a circle circles overlap where there is a covalent bond electrons from one atom are.

Dot Cross Product Practice Problem YouTube
Dot and cross diagrams allow us to visualize how electrons are shared through out a molecule and from which atoms they originated, but do not tell us anything about the shape of those molecules.
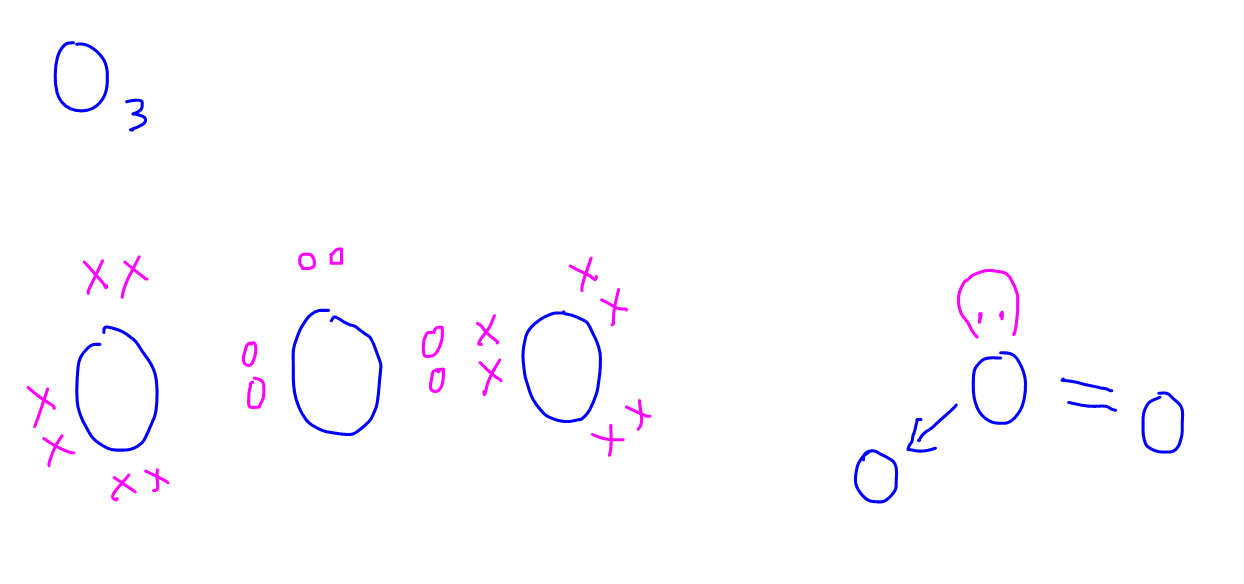
Fluorine Molecule Dot And Cross Diagram Diagram Media
Dot & Cross Diagrams. Dot and cross diagrams are diagrams that show the arrangement of the outer-shell electrons in an ionic or covalent compound or element. The electrons are shown as dots and crosses. In a dot and cross diagram: Only the outer electrons are shown. The charge of the ion is spread evenly which is shown by using brackets.
"Stunning 4K Collection of Over 999 Cross Images The Best Selection"
When drawing a dot-and-cross diagram for covalent bonds you must remember: only the outershell electrons (also called valence electrons) need to be drawn (unless otherwise stated) each covalent bond is a pair of electrons (one electron from each atom in the bond) there can be double bonds (4 shared electrons), or even triple bonds (6 shared.

DOT & CROSS
Topic Covalent bonding and dot and cross diagrams Level GCSE (or any other course for students aged 11-16) Outcomes 1. To understand how a covalent bond is formed 2. To be able to use molecular and displayed formula 3. To draw dot and cross diagrams for simple covalent molecules involving single, double and triple bonds
Dot and Cross Diagram
In this video, we are going to see how to draw dot and cross diagrams for covalent bonding. Specifically, we are going to draw dot and cross diagrams for H2O, NH3, CH4 and CO2. The dot.